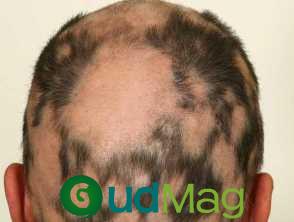
Recently, the Journal of the European Academy of Dermatology and Venereology published combined data from the BRAVE-AA1 (NCT03570749) and BRAVE-AA2 (NCT03899259) trials, which showed the effectiveness and safety of baricitinib for individuals with severe alopecia areata (AA) for a period of 104 weeks.1
According to Senna et al.1, Baricitinib has been approved as the initial systemic remedy for severe alopecia areata (AA) in adults. It has already shown promising results in promoting hair growth in the scalp, eyebrows, and eyelashes, with continued improvement seen throughout 52 weeks.2-3 However, while the long-term effects of JAK inhibitors for AA are not fully known, data from the 52-week follow-up of BRAVE-AA1 and BRAVE-AA2 suggest that extended treatment may be necessary in order to achieve maximum benefit and sustain clinical response.
To assess the effectiveness of baricitinib in the treatment of AA over an extended period, scientists combined data from the BRAVE-AA1 and BRAVE-AA2 studies, both of which administered a consistent dose for 104 weeks to patients with AA.
Ongoing trials called BRAVE-AA1 and BRAVE-AA2 are currently being conducted to evaluate the effectiveness and safety of baricitinib in treating AA. These trials follow a double-blind, parallel-group, and placebo-controlled design. The participants who are eligible, as stated in previously published trial protocols, are adults who have experienced AA for a period of 6 months to 8 years and have Severity of Alopecia Tool (SALT) scores of ≥50, or significant hair loss on the scalp.
The main focus of the efficacy analysis was on patients who were initially assigned to either receive 2 mg or 4 mg of baricitinib and maintained the same dose for 104 weeks. This group consisted of patients who reached a SALT score of ≤20 at week 52 and continued with their current dosage.
For individuals who have a SALT score of more than 20 at week 52, the effectiveness assessments were expanded to include patients who have been consistently using 4 mg of baricitinib since the beginning of the study and have continued this dosage until at least week 76. This was done under the condition that they fulfilled specific requirements for regrowth of eyebrows and/or eyelashes or attained a SALT score of 20 or less.
The findings showed that those who responded positively at week 52 continued to maintain their effectiveness, with most of them having SALT scores ≤20 and experiencing significant enhancements in hair regrowth and quality of life measures at week 104. Both the groups receiving 2 mg and 4 mg doses showed consistent improvements in their anxiety and depression scores throughout the entire duration of the study.
As for those who responded with a mix of positive and negative outcomes in week 52, and had more severe disease characteristics initially, there was a noticeable improvement over time, albeit not as significant as the week-52 responders. However, a considerable number of patients still achieved clinically meaningful results and saw improvements in their quality of life indicators by week 104.
There were no reported or observed signs of new safety concerns.
According to Senna et al.1, long-term treatment may be necessary for controlling the chronic and recurrent nature of severe AA. The use of JAK inhibitors has been a significant development in managing AA, although there is a lack of long-term data in this area. In our study, we present the results of continuous baricitinib treatment over a period of 104 weeks, demonstrating its effectiveness and safety in promoting hair regrowth and improving the quality of life of both Week-52 responders and mixed responders. The number of discontinuations was minimal and there were no new safety concerns identified during the extended observation period. Additional studies, BRAVE-AA1 and BRAVE-AA2, are currently ongoing and will continue to monitor patients for up to 200 weeks.